A novel approach to C1 homologation of carboxylic acids
27 February 2024
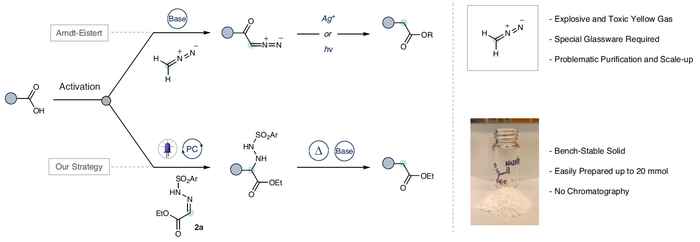
By utilizing ethyl glyoxalate-derived sulfonyl hydrazone as a stable radical acceptor, the team was able to achieve C1 homologation under mild conditions, eliminating the need for hazardous reagents like diazomethane. This breakthrough enables a streamlined synthesis process, exemplified by the efficient preparation of β-arylethylamines—a critical structural motif in pharmaceuticals and natural products. Additionally, the methodology facilitates the late-stage functionalization of peptides, simplifying peptide modification with its straightforward approach.
The research was carried out in a collaboration between the Flow Chemistry group at the University of Amsterdam and researchers at the Institut de Chimie des Substances Naturelles, CNRS, Univ. Paris-Saclay (Gif-sur-Yvette, Cedex, France), the SynCat Lab at the University of Parma (Italy), the PhotoGreen Lab at the University of Pavia (Italy), the BioPharmaceuticals R&D department of AstraZeneca (Gothenburg, Sweden) and the Organic Semiconductor Centre at the University of St Andrews (United Kingdom). This collaborative effort marks a significant step forward in synthetic chemistry, promising safer and more efficient chemical transformations for a variety of applications.
Abstract of the paper
In contemporary drug discovery, enhancing the sp3-hybridized character of molecular structures is paramount, necessitating innovative synthetic methods. Herein, we introduce a deoxygenative cross-electrophile coupling technique that pairs easily accessible carboxylic acid-derived redox-active esters with aldehyde sulfonyl hydrazones, employing Eosin Y as an organophotocatalyst under visible light irradiation. This approach serves as a versatile, metal-free C(sp3)−C(sp3) cross-coupling platform. We demonstrate its synthetic value as a safer, broadly applicable C1 homologation of carboxylic acids, offering an alternative to the traditional Arndt-Eistert reaction. Additionally, our method provides direct access to cyclic and acyclic β-arylethylamines using diverse aldehyde-derived sulfonyl hydrazones. Notably, the methodology proves to be compatible with the late-stage functionalization of peptides on solid-phase, streamlining the modification of intricate peptides without the need for exhaustive de-novo synthesis.
Paper details
Bonciolini, S., Pulcinella, A., Leone, M. et al. Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes. Nat Commun 15, 1509 (2024). DOI: 10.1038/s41467-024-45804-z
See also
Research group Flow Chemistry