Encapsulated rhodium catalyst yields branched aldehydes via hydroformylation of terminal alkenes
27 February 2024
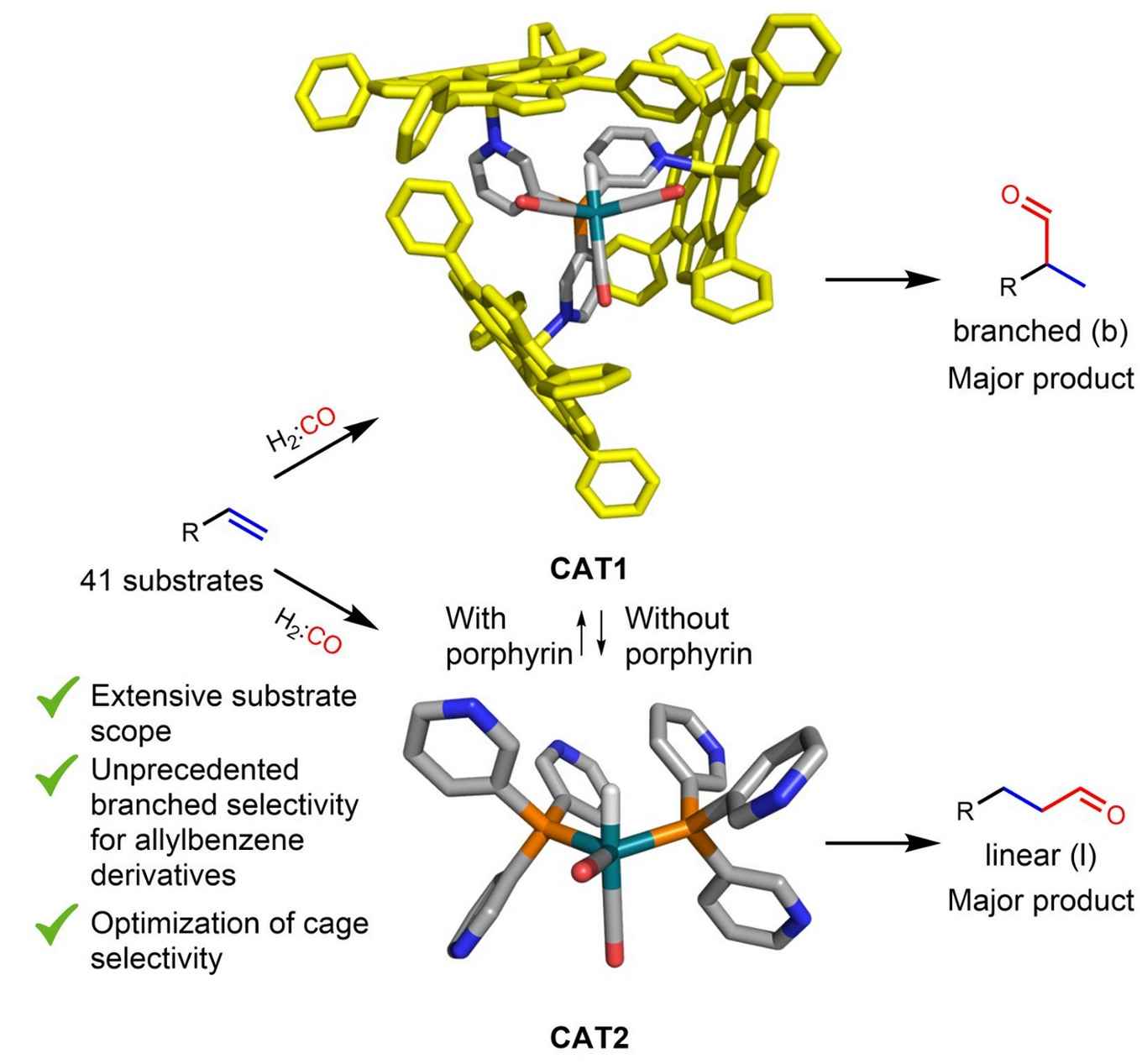
Obtaining branched aldehydes from terminal alkenes has long been a notorious challenge in the field of hydroformylation catalysis. Linnebank and his supervisors Prof. Joost Reek and Dr Sander Kluwer took up this challenge by encapsulating a rhodium catalyst in a porphyrin-based supramolecular cage. They reacted 41 terminal alkenes with this caged catalyst to target branched aldehydes in the hydroformylation reaction. By varying the porphyrin building blocks, they were able to further enhance the selectivity towards the desired branched products.
Abstract of the paper
Caged complexes can provide impressive selective catalysts. Due to the complex shapes of such caged catalysts, however, the level of selectivity control of a single substrate cannot be extrapolated to other substrates. Herein, the substrate scope using 41 terminal alkene substrates is investigated in the hydroformylation reaction with the encapsulated rhodium catalyst [Rh(H)(CO)3(P(mPy3(ZnTPP)3))] (CAT1). For all substrates, the amount of branched product formed was higher with CAT1 than with the unencapsulated reference catalyst [Rh(H)(CO)2(P(mPy3))2](CAT2) (linear/branched ratio between 2.14 and 0.12 for CAT1 and linear/branched ratio between 6.22 and 0.59 for CAT2). Interestingly, the level of cage induced selectivity depends strongly on the substrate structure that is converted. Analysis of the substrate scope combined with DFT calculations suggest that noncovalent interactions between the substrate moieties and cage walls play a key role in controlling the regioselectivity. Consequently, these supramolecular interactions were further optimized by replacing the ZnTPP building block with an zinc porphyrin analog that contained OiPr substituents on the meta position of the aryl rings. The resulting caged catalyst, CAT4, converted substrates with even higher branched selectivity.
Paper details
Pim Linnebank, Sander Kluwer and Joost N. H. Reek: A Substrate Scope Driven Optimization of an Encapsulated Hydroformylation Catalyst Catal. Sci. Technol., 2024, accepted 16 Feb 2024 DOI: 10.1039/D4CY00051J
See also
Research group Homogeneous, Supramolecular and Bio-inspired Catalysis