One-step ethylene purification from quaternary mixtures
6 February 2024
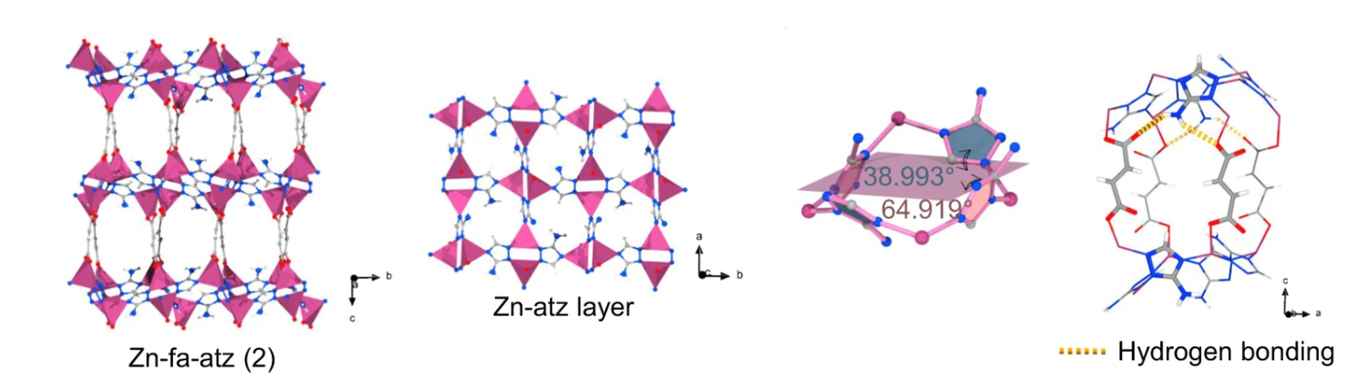
The paper describes how the ultramicroporous adsorbent Zn-fa-atz (2) – where fa stand for fumaric acid and atz for 3-amino-1,2,4-triazolate – can be used for effective one-step ethylene production from the quaternary CO2/C2H2/C2H4/C2H6 mixture. Based on the experimental and simulated breakthrough data, it reports how the Zn-fa-atz (2) coordination network synchronously weakens the affinity for three C2 hydrocarbons, including C2H4, while enhancing CO2 adsorption due to optimized CO2-host interaction and faster CO2 diffusion.
The paper concludes that precise control of the adsorption selectivity of C2H4 in the complex separation systems can be achieved through fine-tuning of the size and shape of the pores, and of the local pore chemistry. The presented design principles could be helpful to advance the synthesis and application of this new-generation physisorbent for more complex industry-related separation systems.
Abstract of the paper
Purification of ethylene (C2H4) as the most extensive and output chemical, from complex multi-components is of great significance but highly challenging. Herein we demonstrate that precise pore structure tuning by controlling the network hydrogen bonds in two highly-related porous coordination networks can shift the efficient C2H4 separation function from C2H2/C2H4/C2H6 ternary mixture to CO2/C2H2/C2H4/C2H6 quaternary mixture system. Single-crystal X-ray diffraction revealed that the different amino groups on the triazolate ligands resulted in the change of the hydrogen bonding in the host network, which led to changes in the pore shape and pore chemistry. Gas adsorption isotherms, adsorption kinetics and gas-loaded crystal structure analysis indicated that the coordination network Zn-fa-atz (2) weakened the affinity for three C2 hydrocarbons synchronously including C2H4 but enhanced the CO2 adsorption due to the optimized CO2-host interaction and the faster CO2 diffusion, leading to effective C2H4 production from the CO2/C2H2/C2H4/C2H6 mixture in one step based on the experimental and simulated breakthrough data. Moreover, it can be shaped into spherical pellets with maintained porosity and separation performance.
Paper details
Yang, R., Wang, Y., Cao, JW. et al.: Hydrogen bond unlocking-driven pore structure control for shifting multi-component gas separation function. Nat Commun 15, 804 (2024). DOI: 10.1038/s41467-024-45081-w