Origins of the increased efficiency of a chimeric styrene monooxygenase
14 March 2024
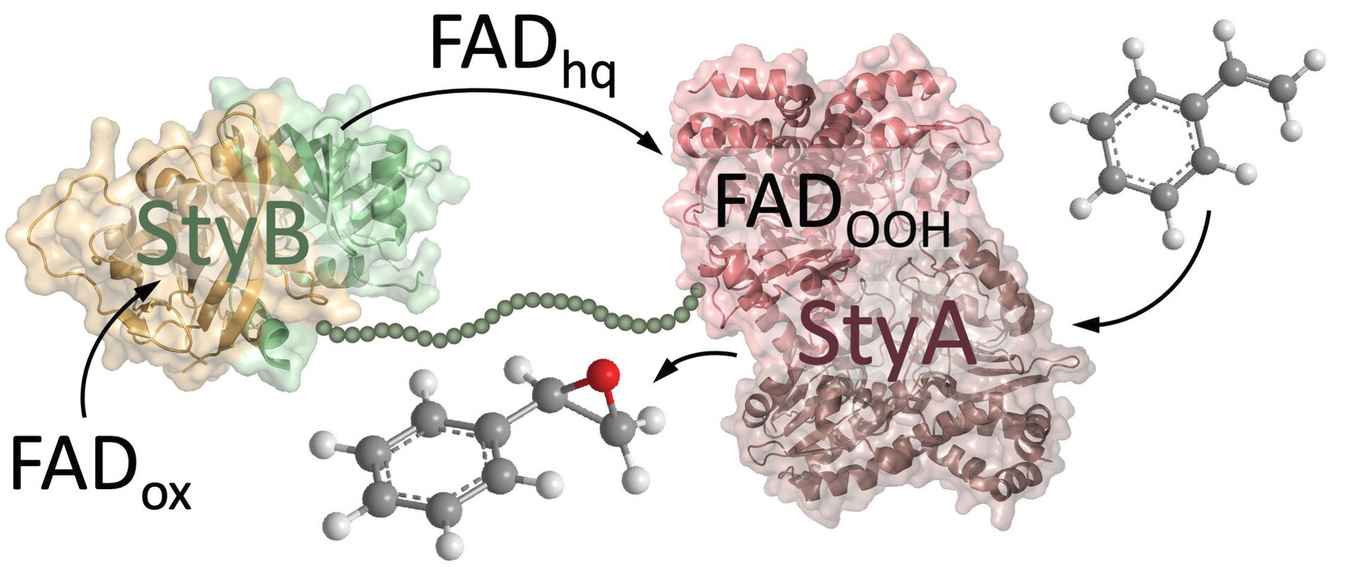
The chimeric styrene monooxygenase (Fus-SMO) was developed earlier by the Biocatalysis group through genetic fusion of two enzymatic units, namely StyA and StyB. This enzymes’ fusion proved useful for asymmetric biocatalytic epoxidation, displaying an increased efficiency over the natural two component StyA/StyB system. Now, Dr Tanja Knaus and Prof. Francesco Mutti, in cooperation with Prof. Peter Macheroux from Graz University of Technology (Austria) investigated the origin of this increased efficiency. They performed pre-steady-state kinetics, studies on NADH consumption vs. styrene epoxidation (i.e. coupling efficiency) and in silico modelling.
The research elucidates important aspects of the transfer of the FAD cofactor between the two components of the SMO - a process that could proceed differently when the two discrete units are isolated in solution or in a cellular environment. They expect that the proximity of StyA and StyB in solution via the fusion with the flexible linker might better replicate cellular conditions. The study also demonstrates how the relatively simple protein engineering technique of protein fusion can significantly improve the catalytic efficiency of a two-component system like SMO, even though the natural system is already applied in chemical synthesis in both laboratory and industry settings.
Abstract of the paper
The styrene monooxygenase, a two-component enzymatic system for styrene epoxidation, was characterised through the study of Fus-SMO – a chimera resulting from the fusion of StyA and StyB using a flexible linker. Notably, it remains debated whether the transfer of FADH2 from StyB to StyA occurs through diffusion, channelling, or a combination of both. Fus-SMO was identified as a trimer with one bound FAD molecule. In silico modelling revealed a well-distanced arrangement (45–50 Å) facilitated by the flexible linker‘s loopy structure. Pre-steady-state kinetics elucidated the FADox reduction intricacies (kred=110 s−1 for bound FADox), identifying free FADox binding as the rate-determining step. The aerobic oxidation of FADH2 (kox=90 s−1) and subsequent decomposition to FADox and H2O2 demonstrated StyA′s protective effect on the bound hydroperoxoflavin (kdec=0.2 s−1) compared to free cofactor (kdec=1.8 s−1). At varied styrene concentrations, kox for FADH2 ranged from 80 to 120 s−1. Studies on NADH consumption vs. styrene epoxidation revealed Fus-SMO′s ability to achieve quantitative coupling efficiency in solution, surpassing natural two-component SMOs. The results suggest that Fus-SMO exhibits enhanced FADH2 channelling between subunits. This work contributes to comprehending FADH2 transfer mechanisms in SMO and illustrates how protein fusion can elevate catalytic efficiency for biocatalytic applications.
Paper details
Tanja Knaus, Peter Macheroux, and Francesco G. Mutti: Fus-SMO: Kinetics, Biochemical Characterisation and In Silico Modelling of a Chimeric Styrene Monooxygenase Demonstrating Quantitative Coupling Efficiency. ChemBioChem, First published: 02 February 2024 DOI: 10.1002/cbic.202300833
See also
Research group HIMS BioCatalysis