Photocatalytic functionalization of dehydroalanine-derived peptides in batch and flow
21 March 2024
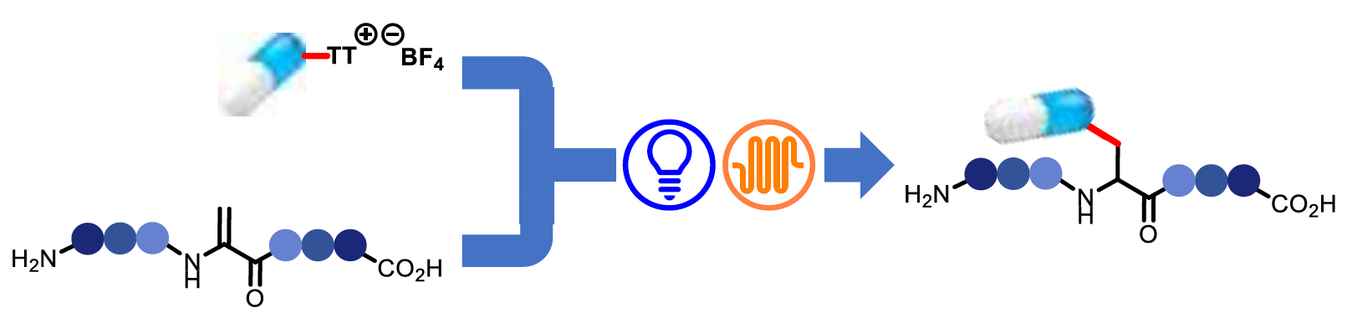
The novel approach offers new avenues for biochemical research and therapeutic development through late-stage functionalization of complex peptides and proteins. It enables the preservation of delicate chemical structures during synthesis, broadening the scope of compatible coupling partners. The precise site- and chemoselectivity of the technique showcases its utility in the modification of peptides bearing a variety of sensitive residues.
Abstract of the paper
Unnatural amino acids, and their synthesis via the late-stage functionalization (LSF) of peptides, play a crucial role in areas such as drug design and discovery. Historically, the LSF of biomolecules has predominantly utilized traditional synthetic methodologies that exploit nucleophilic residues, such as cysteine, lysine or tyrosine. In this study, we present a photocatalytic hydroarylation process targeting the electrophilic residue dehydroalanine (Dha). This residue possesses an α,β-unsaturated moiety and can be combined with various arylthianthrenium salts, both in batch and flow reactors. Notably, the flow setup proved instrumental for efficient scale-up, paving the way for the synthesis of unnatural amino acids and peptides in substantial quantities. Our photocatalytic approach, being inherently mild, permits the diversification of peptides even when they contain sensitive functional groups. The readily available arylthianthrenium salts facilitate the seamless integration of Dha-infused peptides with a wide range of arenes, drug blueprints, and natural products, culminating in the creation of unconventional phenylalanine derivatives. The synergistic effect of the high functional group tolerance and the modular characteristic of the aryl electrophile enables efficient peptide conjugation and ligation in both batch and flow conditions.
Paper details
Nikolaos Kaplaneris, Merve Akdeniz, Méritxell Fillols, Francesca Arrighi, Fabian Raymenants, Gana Sanil, Daniel T. Gryko, Timothy Noel: Photocatalytic Functionalization of Dehydroalanine-Derived Peptides in Batch and Flow. Angewandte Chemie International Edition, e202403271, 18 March 2024. DOI: 10.1002/anie.202403271